
views
Do not confuse this process with acid staining, which colors the concrete. An acid wash is not recommended before applying a stain.[1]
X
Research source
Setting Up

Remove dirt and grease. Brush or vacuum dirt from the concrete. If there are oil stains, remove them with a concrete degreaser or alkali detergent. Rinse thoroughly with water. If water beads up on the surface, the acid wash may not work correctly. Degreasing should solve this problem. Trisodium phosphate (TSP) cleaners are not recommended. Any residue left behind can react violently with the acid to release dangerous gas.
Choose your acid. Select a cleaning or etching product based on your experience level and project location: Sulfamic acid is the safest to handle, and recommended for non-professionals. Phosphoric acid creates less fumes. Use it in rooms that contain stainless steel or other acid-vulnerable metals. It's also a good choice if you're just cleaning off mineral deposits. Muriatic acid (a mixture of hydrochloric acid and cyanide) is the most dangerous option and produces strong fumes. Recommended only for professionals working outdoors.

Follow safety instructions. These acids are some of the most dangerous substances available for home use. Wear acid-resistant gloves, rubber boots, and vapor proof goggles. Protect your lungs with a respirator with an acid-grade filter, and use fans to improve ventilation if necessary. Protect exposed skin with well-fitting clothing at minimum, and ideally with a face shield plus a PVC or Butyl coverall or apron. Keep water nearby to wash spills from skin or clothing. Showers and an eye wash station are ideal. Keep baking soda or garden lime nearby to neutralize spills on the ground.

Add acid to water in a plastic bucket or watering can. Unlike metal, all common plastics are resistant to acid damage at these concentrations. To avoid a violent reaction, pour water in the bucket first, then add acid slowly. Defer to the manufacturer’s directions on the acid container over the guidelines below. These general ratios are suited to some mixes but not all: Sulfamic acid: 1 pound powder or crystals per 1 gallon hot water (120 grams per 1 L water). Phosphoric acid: dilute to 20–40%. Muriatic acid: mix 3 to 4 parts water with 1 part acid, or follow label instructions for a 10% concentration (15% for hard, smooth concrete). These solutions are for etching the concrete. If you're just removing mineral deposits (efflorescence), use a much weaker mix (10:1 or 16:1 for muriatic acid).
Applying the Acid

Hose down the entire area. Spray water on the concrete until damp, but not puddling. Also wet surrounding objects, such as trees, bushes, walls, doors, door frames, cabinetry, and carpets. Remove any furniture in close proximity. The concrete must stay wet the whole time. Divide large areas into sections or hose periodically to prevent it drying out. Protect asphalt, drywall, and tarmac with a plastic drop cloth or other physical barrier.
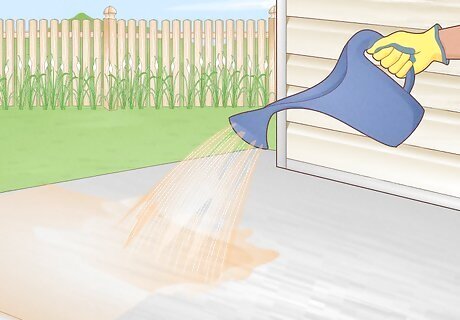
Sprinkle on the acid. Use a plastic watering can to sprinkle acid onto the concrete, pouring low to the ground. Work in small sections, starting with an inconspicuous test area. The plastic may corrode, sometimes within an hour, so have several replacement cans handy.. Read the label instructions to determine how much acid to add, or use these guidelines: Sulfamic acid: 1 gallon treats 300 ft. concrete (1 liter per 28 m). Phosphoric acid: 1 gallon treats 500–2500 ft. (3.8 L per 45–250 m) when removing mineral deposits. Muriatic acid: 1 gallon treats 45 ft. (4.5 L per 5 m).
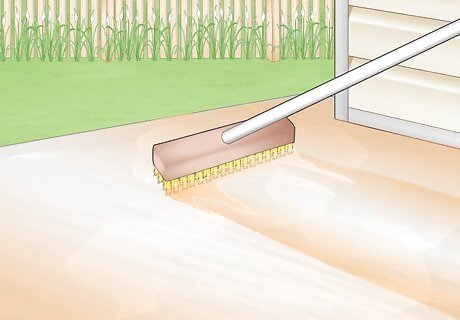
Brush the acid over the concrete. Right after sprinkling on the acid, brush with a push broom or long masonry brush to spread the acid into an even layer. For large jobs, one person can use a floor machine while another brushes acid into the corners and walls. Make sure the floor and surrounding objects don't dry out while you're applying acid. You may need to hose them down frequently.

Let the acid sit for a few minutes. Wait 5–10 minutes for the acid to etch the concrete. If you're just removing white mineral deposits, wait until you see them lift off the concrete (usually just a couple minutes).
Cleaning Up
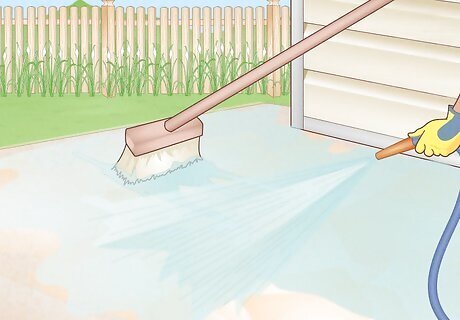
Rinse thoroughly. Before the acid dries, scrub off the remaining residue with a long masonry brush while rinsing with plenty of water. Leaving the acid on too long may damage your concrete.
Neutralize the acid. Mix 1 cup of baking soda, garden lime, or household ammonia in 1 gallon of water (roughly 250 mL in 4 L), or follow label instructions on an acid neutralizing product. Scrub this over the concrete and let sit at least ten minute to ensure that all the acid is neutralized. Pay particular attention to the edges and any low spots in the concrete. At this point, etched concrete should have a uniform texture like medium-grit sandpaper. If the concrete is smoother than this, or if there are still white mineral deposits, apply the acid a second time.

Rinse several times. Even after the acid is neutralized, liquid left on the surface may leave a white, powdery residue after it dries. Spray the concrete with water, scrub, and repeat several times to prevent this from happening. Pick up the final rinse water with a shop vac or brush it into the gutter. Use a hose to rinse the acid rather than a pressure washer. These can drive acid deep into the concrete. To be safe, test the final rinse water with a pH test strip. If it is below 6.0, there is too much acid and the floor needs more rinsing. (Less commonly, a result above 9.0 means you've added too much basic neutralizer.)
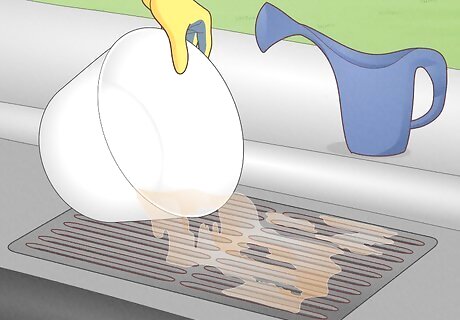
Clean up leftover acid. If you have any leftover acid solution, pour it slowly into a large bucket filled partway with the same neutralizing solution you used earlier. Slowly stir in more acid and base as needed until the mixture stops fizzing. Once neutralized, you can pour it down the sink or storm drain. Hose off any equipment or clothing that may have touched the acid. If you do not have plans for the rest of the pure acid, you may want to dispose of it the same way. Acid left in storage can be a serious hazard due to corrosive fumes and the risk of spills.




















Comments
0 comment