views
New Delhi: Dr. Reddy’s Laboratory is not producing any generic copy of the antiviral drug Remdesivir as it is a patented drug, Managing Director G V Prasad told CNBC TV18.
He also said the company was not in the process of conversation with Remdesivir maker Gilead Sciences on the issue.
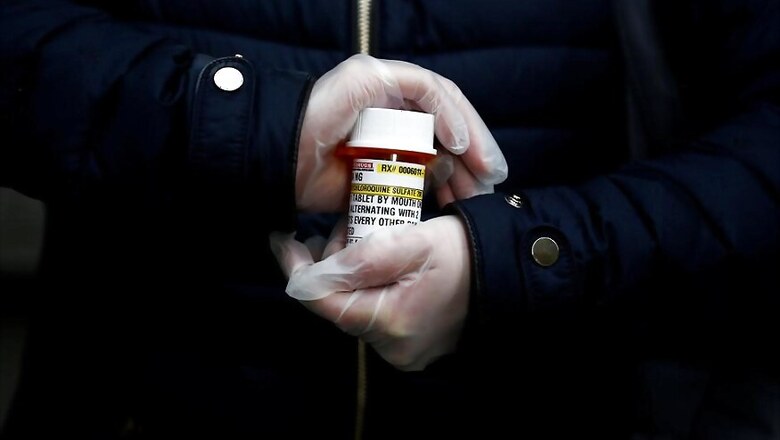
Prasad said that Dr Reddy’s is producing the anti-malarial drug Hydroxychloroquine for the United States and that their facilities US were fully operational.
“We have not commenced manufacturing of hydroxychloroquine in India but we are producing it in the US. There is some shortage of raw materials for making HCQ but there is no scarcity of the drug in India,” Prasad told CNBC TV18.
“Manufacturing of HCQ shouldn’t be a problem as many Indian companies are making the required APIs. All our manufacturing sites in India are now operational at steady utilisation levels," Prasad added.
GV Prasad said that Dr Reddy’s Laboratories had not begun any specific work on a remedy for the novel coronavirus disease.
The antiviral drug Remdesivir was developed by US Biotech company Gilead Sciences, was used on Ebola patients in limited trials in Africa.
Earlier in a government briefing, Indian Council of Medical Research said that there have been early reports of Covid-19 patients showing improvements after Remdesivir was given during treatment.
Patients did not require ventilator support after Remdesivir treatment, during the trials, ICMR scientists had said. However, ICMR is awaiting developments of the World Health Organisation’s Solidarity Trial.
Remdesivir was previously tested in animal studies for Middle East Respiratory Syndrome (MERS) and severe acute respiratory syndrome (SARS). Both infectious diseases were caused by coronaviruses.
















Comments
0 comment