views
Writing Chemical Formulas of Covalent Compounds

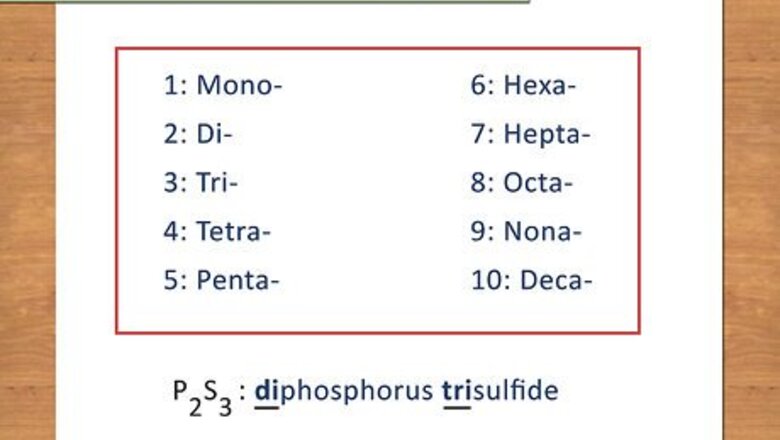
Memorize the prefixes for number of atoms. In naming compounds, Greek prefixes are used to indicate the number of atoms present for each element. Covalent compounds are written out as molecular formulas due to the fact that each compound is a distinct, separate molecule. Covalent compounds have the first element written out completely while the second element is named with the suffix “ide.” For example, diphosphorus trisulfide has a chemical formula of P2S3. Below are the prefixes for 1-10: 1: Mono- 2: Di- 3: Tri- 4: Tetra- 5: Penta- 6: Hexa- 7: Hepta- 8: Octa- 9: Nona- 10: Deca-

Write the chemical symbol for the first element. When a compound has been written out, you must identify the elements and know their chemical symbols. The first element written is “first name” of the compound. Use the periodic table to find the chemical symbol for the element. For example: Dinitrogen hexafluoride. The first element is nitrogen and the chemical symbol for nitrogen is N.
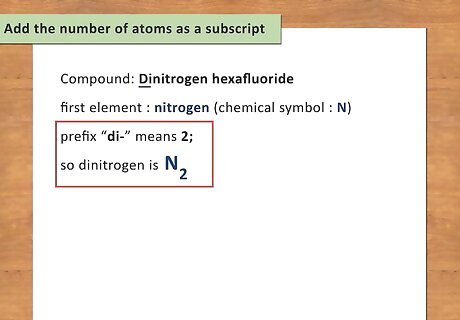
Add the number of atoms as a subscript. To identify the number of atoms present for each element, you simply need to look at the prefix of the element. Memorizing the Greek prefixes will help you to be able to write chemical formulas quickly without looking anything up. For example: Dinitrogen has a the prefix “di-“ which means 2; therefore, there are 2 atoms of nitrogen present. Write dinitrogen as N2.
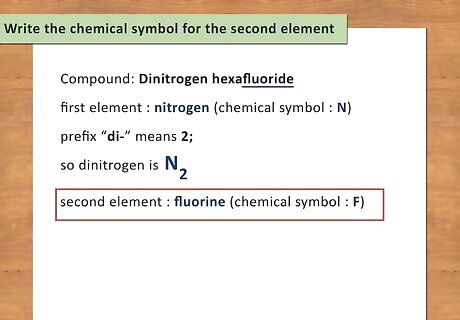
Write the chemical symbol for the second element. The second element is the “last name” of the compound and will follow the first element. For covalent compounds, the element name will have a suffix of “-ide” instead of the normal ending of the element. For example: Dinitrogen hexafluoride. The second element is fluorine. Simply replace the “ide” ending with the actual element name. The chemical symbol for fluorine is F.
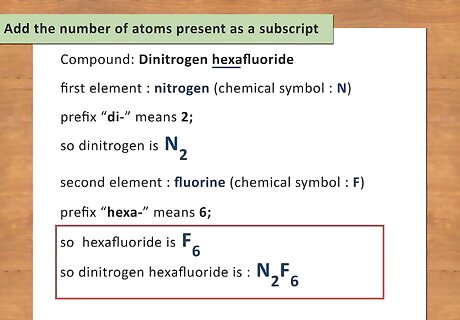
Add the number of atoms present as a subscript. As you did with the first element, identify the number of atoms present in the second element by reading the prefix. Using this prefix, write the number of atoms as a subscript to the right of the chemical symbol. For example: Hexafluoride has a prefix of “hexa-“ which means 6; therefore, there are 6 atoms of fluorine present. Write hexafluoride as F6. The final chemical formula for dinitrogen hexafluoride is N2F6.
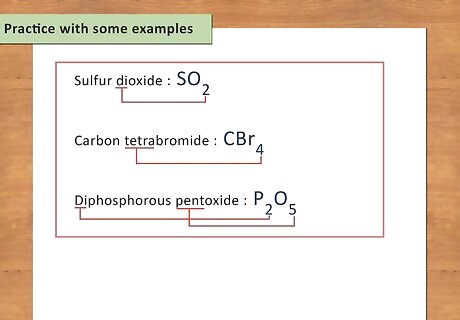
Practice with some examples. When first learning chemistry, there is a lot of memorization involved. It is kind of like learning a new language. The more examples you practice with, the easier it will be to decipher chemical formulas in the future and learn the language of chemistry. Sulfur dioxide: SO2 Carbon tetrabromide: CBr4 Diphosphorus pentoxide: P2O5
Writing Chemical Formulas of Ionic Compounds
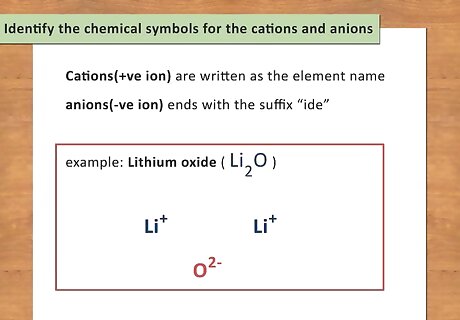
Identify the chemical symbols for the cations and anions. All chemicals have what you can call a first and last name. The first name is the cation (positive ion) while the last name is the anion (negative ion). Cations are written as the element name while anions are the element name ending with the suffix “ide.” The chemical symbol for each element can be found on the periodic table. Unlike covalent compounds, Greek prefixes are not used to indicate the number of atoms of each element. You have to balance the charges of the elements to determine the atoms. For example: Lithium oxide is Li2O.
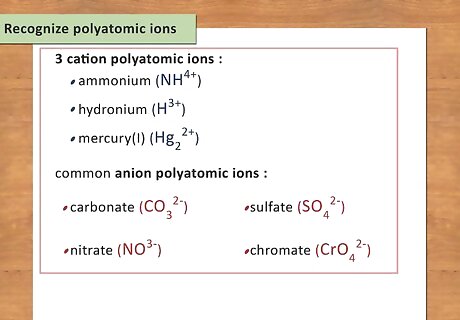
Recognize polyatomic ions. Sometimes the cation or anion is a polyatomic ion. These are molecules that have two or more atoms with ionic groups. There’s no good trick to remembering these, you just need to memorize them. There are only 3 cation polyatomic ions and they are ammonium (NH4), hydronium (H3), and mercury(I) (Hg2). They all have a +1 charge (though, technically, 2 mercury atoms are bonded together, which creates a 2+ charge, with each mercury cation containing a 1+ charge). The rest of the polyatomic ions have negative charges ranging from -1 to -4. Some common ones are carbonate (CO3), sulfate (SO4), nitrate (NO3), and chromate (CrO4).

Determine the valence charge of each element. The valence charge can be determined by looking at the position of the element on the periodic table. There are a few rules to keep in mind that help you identify the charges: All group 1 elements at +1. All group 2 elements are +2. Transition elements will have Roman numerals in parentheses to indicate their charge. Silver is 1+, zinc is 2+, and aluminum is 3+. Group 17 elements are 1-. Group 16 elements are 2-. Group 15 elements are 3-. Remember, when working with polyatomic ions, use the charge of the complete polyatomic ion, rather than the individual ions.
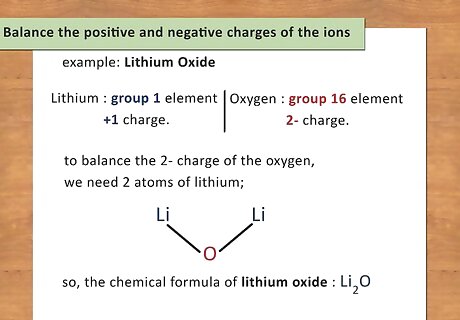
Balance the positive and negative charges of the ions. Once you have identified the charge of each element (or polyatomic ion), you will use these charges to determine the number of atoms present of each element. You want the charge of the compound to equal zero so you will add atoms to balance the charges. For example: Lithium Oxide. Lithium is a group 1 element and has a +1 charge. Oxygen is a group 16 element and has a 2- charge. In order to balance the 2- charge of the oxygen, you need 2 atoms of lithium; therefore, the chemical formula of lithium oxide is Li2O.

Practice with some examples. The best way to learn formula writing is to practice with lots of examples. Use examples in your chemistry book or look for practice sets online. Do as many as you can until you feel comfortable writing chemical formulas. Calcium Nitride: Symbol for calcium is Ca and symbol of nitrogen is N. Ca is a group 2 element and has a charge of +2. Nitrogen is a group 15 element and has a charge of 3-. To balance this, you need 3 atoms of calcium (6+) and 2 atoms of nitrogen (6-): Ca3N2. Mercury(II) Phosphate: Symbol for Mercury is Hg and phosphate is the polyatomic ion PO4. Mercury has a 2+ charge as indicated by the Roman numeral II next to it. Phosphate has a 3- charge. In order to balance them, you will need 3 atoms of mercury (6+) and 2 molecules of phosphate (6-): Hg3(PO4)2.
Determining the Products Given Reactants
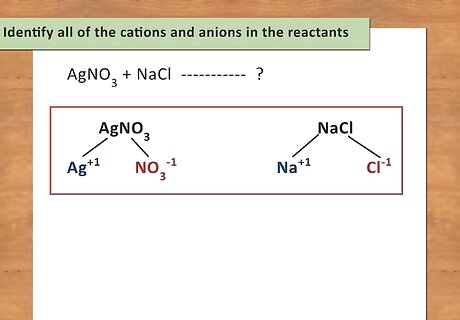
Identify all of the cations and anions in the reactants. In a basic double replacement equation you will have 2 cations and 2 anions. The general equation takes the form of AB + CD → AD + CB, where A and C are cations and B and D are anions. You also want to determine the charges of each ion. For example: AgNO3 + NaCl → ? The cations are Ag and Na. The anions are NO3 and Cl.
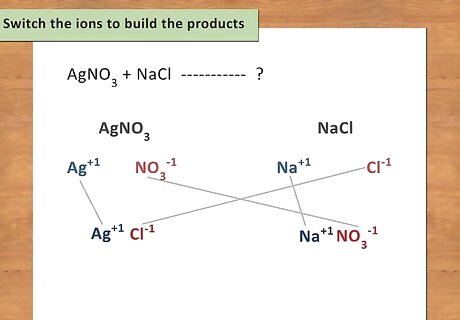
Switch the ions to build the products. Once you have identified all of the ions and their charges, rearrange them so that the first cation is now paired with the second anion, and the second cation is now paired with the first anion. Remember the equation: AB + CD → AD + CB. Remember to balance the charges when forming new compounds. For example: AgNO3 + NaCl → ? Ag now pairs with Cl to form AgCl. Na now pairs with NO3 to form NaNO3.

Write the full equation. After writing the products that will form in the equation, you can write the whole equation with both products and reactants. Keep the reactants on the left side of the equation and write the new products on the right side with a plus sign between them. For example: AgNO3 + NaCl --> ? AgNO3 + NaCl --> AgCl + NaNO3
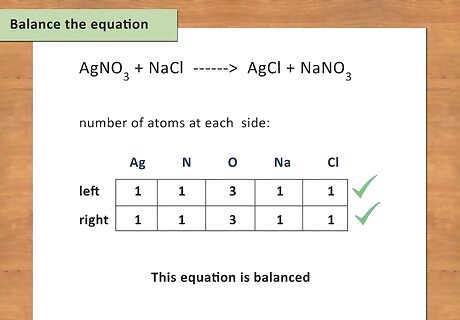
Balance the equation. Once you have written the equation and have all of the products and reactants you need to make sure everything is balanced. An equation is balanced only when you have the same number of atoms of every element present on both sides. For example: AgNO3 + NaCl --> AgCl + NaNO3 Count the number of atoms on each side: 1 Ag left, 1 Ag right; 1 N left, 1 N right; 3 O left, 3 O right; 1 Na left, 1 Na right; 1 Cl left, 1 Cl right This equation is balanced because there are equal numbers of atoms on both the left and right side of the equation.
Note the states of matter. It’s important to indicate the states of matter for both the reactants and the products. There is a designated letter for each state of matter which goes in parentheses. Put this information after the formula of the substance it is describing. Use “(g)” to indicate a gas, “(s)” to indicate a solid, “(l)” to indicate a liquid, and “(aq)” to indicate a substance dissolved in water.
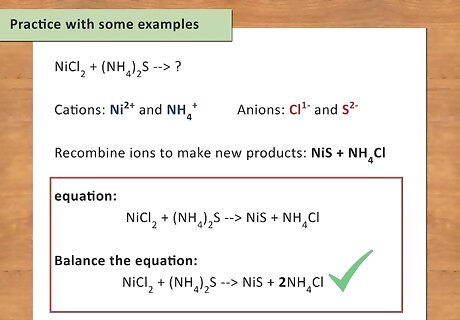
Practice with some examples. The only way to get better at writing chemical equations is to actually do it. Work your way through these examples to make sure you really understand the process. NiCl2 + (NH4)2S → ? Cations: Ni and NH4 Anions: Cl and S Recombine ions to make new products: NiS + NH4Cl Write the equation: NiCl2 + (NH4)2S → NiS + NH4Cl Balance the equation: NiCl2 + (NH4)2S → NiS + 2NH4Cl

















Comments
0 comment