97
views
views
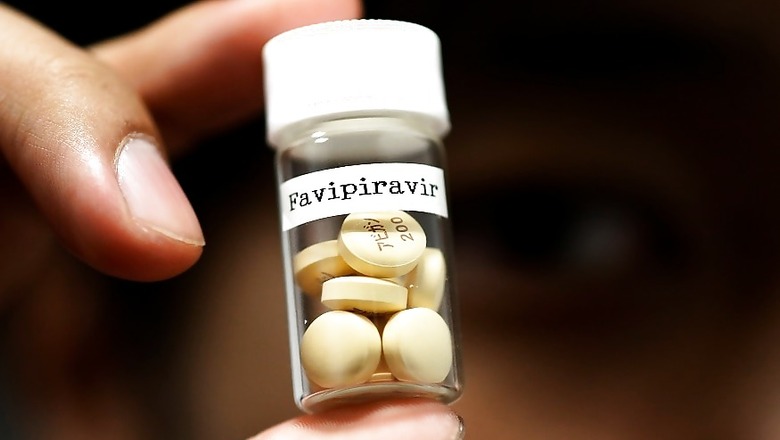
Glenmark Pharma is currently conducting trials for Favipiravir for use on coronavirus patents. While the trials are still underway, the DCGI approved the drug after seeing encouraging results.
The Drug Controller General of India (DCGI) has approved the use of anti-viral drug Favipiravir for COVID-19 patients for restricted use. The drug can be used only in case of an emergency and family consent of the patient is mandatory.
The duration of the course is 14 days.
Glenmark Pharma is currently conducting trials for Favipiravir for use on coronavirus patents. While the trials are still underway, the DCGI approved the drug after seeing encouraging results.
The drug regulator said surveillance for the first 1,000 patients will be needed after administering Favipiravir to them.
While the drug is generally used for influenza around the globe, Russia, China and Japan have approved its usage for COVID-19.

















Comments
0 comment