views
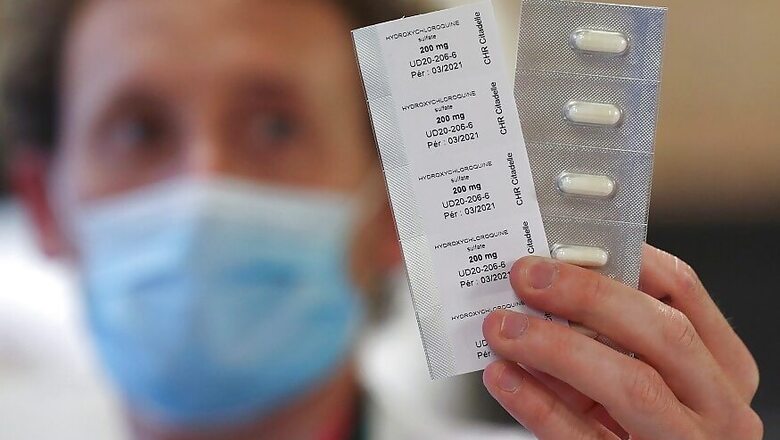
New Delhi/Geneva: The World Health Organisation on Monday temporarily halted trial on anti-malarial drug hydroxychloroquine for treatment of Covid-19, days after the Union health ministry issued an advisory expanding the use of the medicine as a prophylactic to prevent people from contracting the infection.
The steering committee decided to suspend enrollment to that arm of the so-called Solidarity trials, WHO officials. The decision came after the Lancet published a study that said the drug, touted by US President Donald Trump as a treatment, was linked to an increased risk of death and heart ailments.
"This concern relates to the use of hydroxychloroquine and chloroquine in Covid-19," WHO director-general Tedros Adhanom Ghebreyesus said, adding that the drugs are approved treatments for people with malaria or autoimmune diseases. Other treatments in the trial, including the experimental drug remdesivir and an HIV combination therapy, are still being tested.
Tedros said the executive group behind WHO's global 'Solidarity' trial met on Saturday and decided to conduct a comprehensive review of all available data on hydroxychloroquine and that its use in the trial would be suspended for now.
Dr. Michael Ryan, WHO's emergencies chief, said there was no indication of any safety problems with hydroxychloroquine in the WHO trial to date, but that statisticians would now analyze the information.
We're just acting on an abundance of caution based on the recent results of all the studies to to ensure that we can continue safely with that arm of the trial, he said. WHO said it expected to have more details within the next two weeks.
Last week, Trump announced he was taking hydroxychloroquine although he has not tested positive for Covid-19. His own administration has warned the drug can have deadly side effects, and both the European Medicines Agency and the US Food and Drug Administration warned health professionals last month that the drug should not be used to treat Covid-19 outside of hospital or research settings due to numerous serious side effects that in some cases can be fatal.
Hydroxychloroquine and chloroquine are approved for treating lupus and rheumatoid arthritis and for preventing and treating malaria, but no large rigorous tests have found them safe or effective for preventing or treating Covid-19.
(With inputs from AP)


















Comments
0 comment