views
Complex processes, scattered production units, need for more safety zones and shortage of skilled staffers are some of the top reasons that restricted Bharat Biotech’s Covaxin from becoming the poster-boy of India’s vaccination story against Covid-19.
On January 4, Bharat Biotech’s managing director Dr Krishna Ella had said in a virtual press briefing that the company is aiming to manufacture 70 crore doses in 2021.
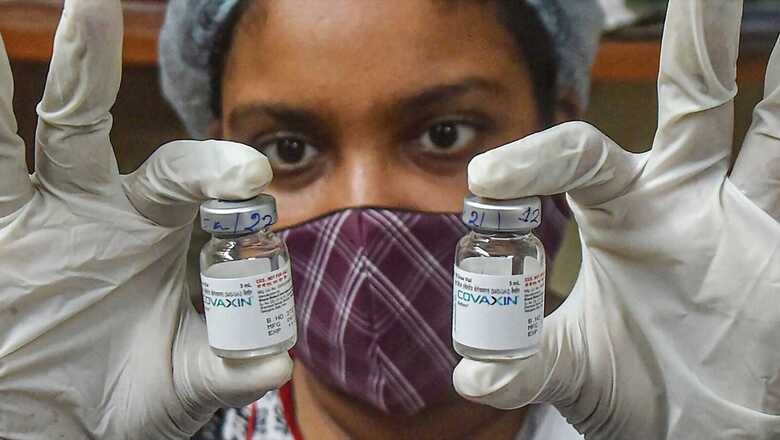
Covaxin, India’s first indigenous vaccine, was touted to play an important role in the country’s massive vaccination drive. However, it turned out to be India’s most expensive vaccine, and that too, available in limited stocks. Meanwhile, Covishield, the vaccine developed by UK’s Oxford University and drug major AstraZeneca, manufactured by Pune-based Serum Institute of India (SII), became the backbone of the campaign.
Out of 81 crore vaccines administered, the SII has supplied over 71.50 crore doses, capturing 88.4% share in India’s vaccination drive. This was supported by 9.28 crore doses of Covaxin, occupying 11.5% share followed by 8.90 lakh doses of Sputnik V, capturing a negligible 0.1% share.
The Union health ministry had informed the Supreme Court that Bharat Biotech initially produced 90 lakh doses every month, which was augmented to 2 crore doses by May. While the company has been trying to boost production, it has failed to meet the promised targets.
In a new set of promises, Bharat Biotech aims to supply 3.5 crore doses of Covaxin in September and 5 crore doses in October.
In an interview with CNBC-TV18, Dr Ella had said, “We are working with other companies. If all of them deliver as we anticipate, we should be able to reach the 10 crore figure by year-end.”
Karisha Ella, MD, @BharatBiotech speaks to CNBC-TV18's @TimsyJaipuria on #Covid vaccine for children. pic.twitter.com/esHBdRg1re? News18 (@CNNnews18) September 1, 2021
Public health experts have raised questions over production claims of the company and allege that the government has extended “extraordinary support” to it.
?The government has consistently overstated the production of Covaxin while the company has made inflated claims about its capacity and readiness of the new production sites. Also, their past and current projections continue to be out of sync with the actual number of doses being manufactured,” said Malini Aisola, convenor of All India Drug Action Network, an NGO.
News18.com takes a look at the host of reasons that worked in favour of Covishield and held Covaxin back from becoming the face of the country’s vaccination drive.
Humongous Task of Scaling Production of Inactivated Virus Platform
”Inactivated vaccines are one of the toughest to produce in the world,” Dr Ella told CNBC-TV18 in an interview. Six experts News18.com spoke to agreed.
These experts include two officials from public sector units that have been given contracts to manufacture Covaxin, an official from one of the top private vaccine makers in India, a former chief of a major vaccine producer who is now a consultant, a scientist working mainly on vaccines and biological products apart from a source from Bharat Biotech. All of them spoke to News18.com on condition of anonymity.
A public sector vaccine maker, which has collaborated with Bharat Biotech to manufacture Covaxin, has been trying to import equipment used in the purification step of the vaccine. “Six months ago, we placed an order which was delivered in 45 days. Right now, for the same equipment’s delivery, the seller needs at least six to eight months,” said an official working with the PSU on kick-starting production of Covaxin at their site. “The order books of all reputed vaccine equipment makers are flooded as the market is small and right now, whole world is trying their luck in vaccine-making.”
Another officer, who is working with a private vaccine manufacturing company, echoed the views. “Setting up new manufacturing facilities, obtaining required regulatory approvals and commissioning, and qualifying them for commercial production is highly resource-intensive and can take around 18-24 months,” he said.
Industry experts also mentioned that the background of two chiefs — SII CEO Adar Poonawalla and Bharat Biotech MD Krishna Ella — could probably be one of the reasons behind the success of Covishield and the erratic supply of Covaxin so far. While Adar is a businessman, Ella is a scientist.
“Adar knows how to conduct himself. His planning, production, targets and supplies were all synchronised. Bharat Biotech suffered from poor planning and lack of futuristic vision apart from working on difficult vaccine platform. We can’t blame Dr Ella fully as he is a scientist and not a well-trained businessman like Adar,” said an official from a second PSU News18.com spoke to. This PSU, too, is upgrading its facilities to manufacture Covaxin.
Human Resources Crunch
Human resources crunch is a reality, specifically for products that deal with live virus and need employees to work in Biosafety Level 3 and Biosafety Level 4 facilities. “Vaccine industry is very small with just 7-8 major players. The talent pool keeps shifting jobs between these players. Right now, everyone is expanding and trying to retain its staff,” said an official from the second PSU quoted above.
He added that “retaining staff in PSUs is one of the biggest challenges as salaries are not attractive. Serum Institute of India is known as one of the best pay masters in the industry.”
Another official, who is an industry veteran and now runs his own consultancy service, said Bharat Biotech needs staffers who are trained to work in facilities dealing with live virus. “It?s a huge challenge as they might not find suitable candidates and it takes a lot of time to attract and train new people to take responsibilities.”
“You may never know when the rival company may poach your talent as you finish their training. All companies, right now, are looking out for trained staffers with best possible salary offers.”
Dr Ella had confirmed in his CNBC-TV18 interview that he is facing talent crunch. He had pointed out that well-trained human resource is critical for the production of Covaxin since there are 200 tests to be done on quality control.
“For all that, you need the best human resources. It’s not easy to just do technology transfer to someone and ask them to produce. It’s not possible because it?s a highly skilled job and most of them don’t have good human resources. So, we are also training people,” he said.
Long, Complex Production Cycle
According to experts, the number of steps to manufacture a vaccine with inactivated virus is much more than ones carried out for adenovirus vector-based vaccines. Covaxin falls in the former category and Covishield in the latter.
According to Bharat Biotech, Covaxin takes 120 days from manufacturing to release.
Sanjiv Navangul, CEO & MD of BSV, a biopharmaceutical company known for its R&D capabilities and its manufacturing facilities both in India and Germany, explained further. “Inactivated vaccines are made from disease-carrying viruses through various treatments using chemicals, heat or radiation. Whole inactivated virus vaccine produced in vero cells is a very complex manufacturing process, involving several steps with high degree of complexity,” he said.
Navangul further added, “Processes include adherent cell culture process, virus inactivation, multiple purification steps, filling of sterile-filtered formulated bulk into vials in aseptic condition etc.?
In a detailed explanation of the timelines involved from manufacturing to vaccination, Bharat Biotech had earlier explained that manufacturing, testing, release and distribution of vaccines is a “complex and multifactorial process”? with hundreds of steps, requiring a diverse pool of human resources.
“There is a four-month lag time for Covaxin to translate into actual vaccination,” the company had said earlier.
As per the company, the timeline for vaccine supplies to reach depots of state and central governments from Bharat Biotech’s facilities is around two days. The vaccines received at these depots have to be further distributed by state governments to various districts within their respective states. “This requires an additional number of days,” the company said in a release.
Challenge of Growing Virus in Vero Cells
The making of Covaxin requires vero cells, which are derived from the kidney of an African green monkey, and are one of the more commonly used cell lines in research and production of vaccines against viruses.
These cells are infected with live virus. Then the virus is harvested and purified. It is then inactivated, or completely killed, so that it stops replication, but is capable of inducing immune response in the human body. Then, the killed virus is formulated and adjuvants are added to boost immune response and its longevity.
“While all steps are crucial for the making of safe and effective vaccines, the inactivation step is critical. Any mistake can lead to serious issues and it has to be proved for every batch that inactivation is complete,” said the former chief of a vaccine-making firm who is now a consultant.
“For adenovirus vector-based vaccine, the process is simple and the virus has to be just grown, harvested, purified and formulated. There is no need to inactivate and other steps are also much simpler.”
Growing of vero cells is another challenge, scientific and industry experts pointed out. “They need a solid surface, for instance, inside glass or plastic bottles and handling them is a laborious process,” the consultant added.
Another way to grow these cells is on micro-carrier beads. This technology, also known as bioreactor cultivation of the vero cells, if works well, gives good results and the infection of the cells can be done and virus grown in big vessels.
“This technology was attempted by the company in its Karnataka-based plant originally making an animal vaccine, but it was probably not executed well and failed to give good results.”
Dr Ella also had said earlier this month that his Malur, Karnataka facility had “let us down a little” but was now “back in action”.
It is also important to understand that results from these new biological process technologies take their own time. “One needs to experiment, learn, improve and then do it again. Sometimes, it takes around a few years only to understand the right way of execution,” the vaccine manufacturer-turned-consultant said.
“I believe that for the Ankleshwar unit, the company is exploring fixed-bed technology. Let?s see how it works for them,” he added.
Too Many Production Units
According to industry experts, Bharat Biotech has split its production into too many units which are based far off from the company’s headquarters in Hyderabad.
“You lose focus when your production is split in too many units located across states and when you are experimenting all the time at plant scale. Dr Ella or his key technical heads cannot be present everywhere. On other hand, SII’s production of Covishield is based in Pune and they have good control over everything from raw material supply, supervision of production, testing and logistics, the consultant explained.
While the company-owned facilities are based in Hyderabad, Karnataka, Pune and Ankleshwar for the production of Covaxin, Bharat Biotech has also signed contracts with three public sector units to enhance production. The units are Bharat Immunologicals and Biologicals Corporation (BIBCOL), Haffkine Bio-Pharmaceutical Corporation, Mumbai, and Indian Immunologicals (IIL), Hyderabad.
“Haffekine has to upgrade itself into a BSL-3 facility whereas BIBCOL also has to upgrade its facility. IIL has started production. Bharat Biotech is working on such a platform (live-virus) where tech-transfer will take a lot of time. It?s not at all easy,” he pointed.
Govt’s Extraordinary Support to Bharat Biotech
Health experts blame the government for lopsided support to Bharat Biotech.
?From supporting development and clinical trials, accelerated regulatory approval, and now manufacturing, Bharat Biotech has benefitted from government backing and even favouritism at all crucial stages,” said public health expert Malini Aisola.
“The government actively enabled Bharat Biotech to retain a monopoly on the vaccine which was co-developed with government institutions,” she said, adding, “Only in the midst of the second wave crisis did the government start hunting for more manufacturing units to make Covaxin.”
“Even though some PSUs have been involved now, the terms of engagement are not transparent and it appears the firms would end up being simply contract manufacturers to serve Bharat Biotech and possibly not as alternative suppliers to the government or private markets,” she further said.
News18.com reached out to Bharat Biotech for comment. However, the company said “Allow us sometime to revert as the team is busy with covid19 vaccine works and public health is the priority right now.” The request for official comments was put up again after a gap of week. However, the company didnot send responses.
Read all the Latest News , Breaking News and Ukraine-Russia War Live Updates here.

















Comments
0 comment