
views
Learn the Fundamentals
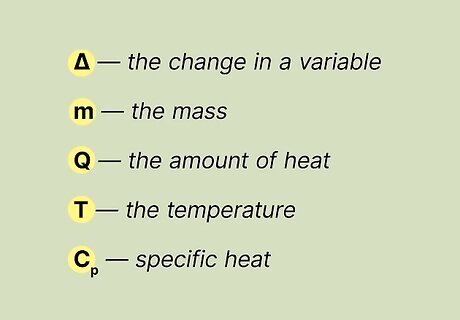
Become familiar with the terms that are used for calculating specific heat. It's important to be familiar with the terms that are used for calculating specific heat before you learn the formula for specific heat. You'll need to know how to recognize the symbol for each term and to understand what it means. Here are the terms that are commonly used in the equation for calculating the specific heat of a substance: Delta, or the "Δ" symbol, represents the change in a variable. For example, if your first temperature (T1) is 150ºC, and your second temperature (T2) is 20ºC, then ΔT, or the change in temperature, represents 150ºC - 20ºC, or 130ºC. The mass of the sample is represented by "m". The amount of heat is represented by "Q". The amount of heat is represented by "J", or Joules. "T" is the temperature of the substance. Specific heat is represented by "Cp".
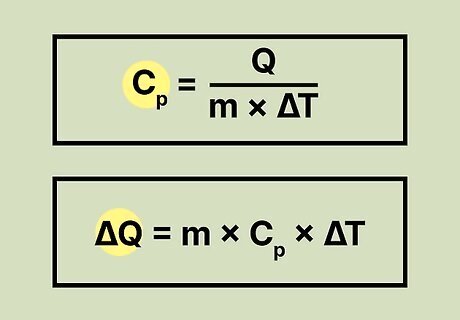
Learn the equation for specific heat. Once you become familiar with the terms used for calculating specific heat, you should learn the equation for finding the specific heat of a substance. The formula is: Cp = Q/mΔT. You can manipulate this formula if you want to find the change in the amount of heat instead of the specific heat. Here's what it would look like: ΔQ = mCpΔT
Calculate Specific Heat
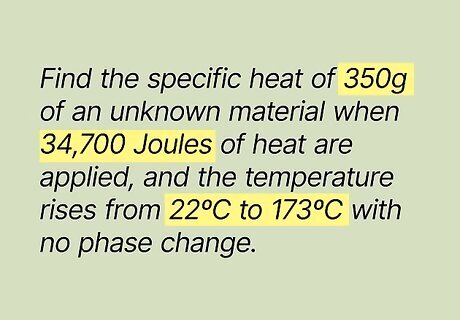
Study the equation. First, you should look at the equation to get a sense of what you need to do to find the specific heat. Let's look at this problem: Find the specific heat of 350 g of an unknown material when 34,700 Joules of heat are applied, and the temperature rises from 22ºC to 173ºC with no phase change.
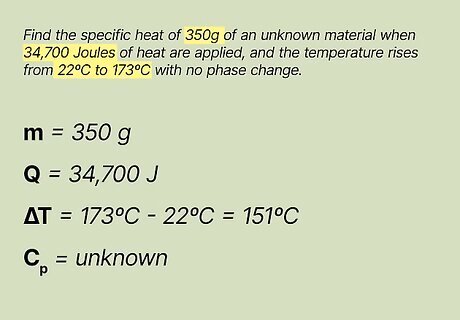
List the known and unknown factors. Once you're comfortable with the problem, you can write down each known and unknown variable to have a better sense of what you're working with. Here's how you do it: m = 350 g Q = 34,700 Joules ΔT = 173ºC - 22ºC = 151ºC Cp = unknown
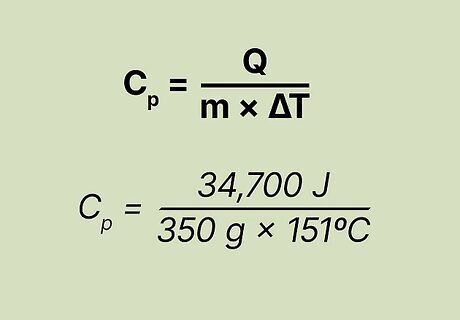
Plug the known factors into the equation. You know the value of everything except "Cpc", so you should plug the rest of the factors into the original equation and solve for "Cp", Here's how you do it: Original equation: Cp = Q/mΔT c = 34,700 J/(350 g x 151ºC)
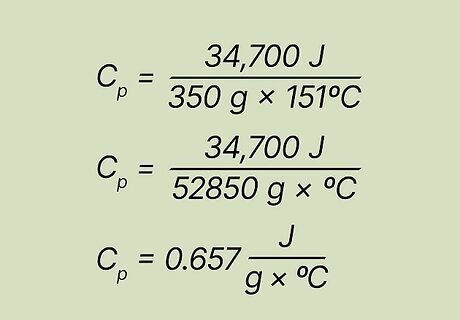
Solve the equation. Now that you've plugged the known factors into the equation, just do simple arithmetic to solve it. The specific heat, or final answer, is 0.65657521286 J/(g x ºC). Cp = 34,700 J/(350 g x 151ºC) Cp = 34,700 J/(52850 g x ºC) Cp = 0.65657521286 J/(g x ºC)

















Comments
0 comment