
views
- Spray a solution of 2 cups (470 mL) hydrogen peroxide, 4 US tbsp (59 mL) white vinegar, and 1.5 tsp (8.5 g) of table salt on a clean piece of metal to rust it.
- Gently rinse the metal in water once it’s as rusted as you want it to be, dry it, then seal it with a coat of polyurethane.
- For galvanized metal, you'll need to strip the zinc coating first using diluted muriatic acid.
How to Rust Metal
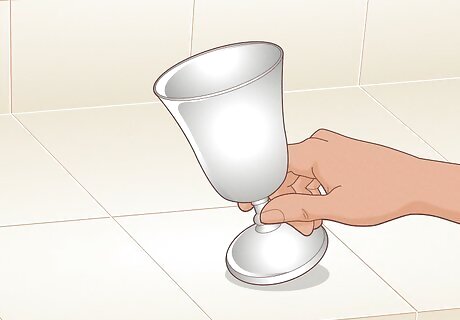
Ensure the metal you’re working with can rust. Only metals containing iron will rust, and some iron alloys rust slowly or not at all. Cast iron or wrought iron rusts the most easily, whereas stainless steel, an alloy of iron and chromium, is more difficult.
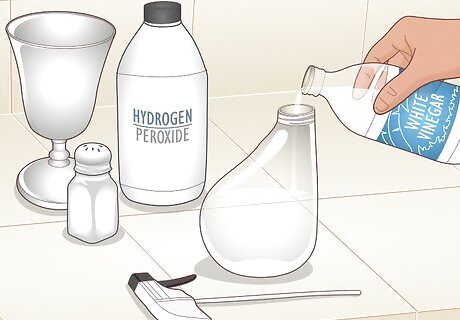
Make a solution of vinegar, hydrogen peroxide, and salt. Mix 2 cups (470 mL) hydrogen peroxide, 4 US tbsp (59 mL) white vinegar, and 1.5 tsp (8.5 g) of table salt in a spray bottle. If you just bought the metal, there may be a thin layer of grease on it. If so, mix hot water with a small amount of liquid dish soap, spray the metal with it, then wipe it off. If you’re using painted metal, remove the paint by sanding it down with coarse sandpaper. If you don’t want to scratch the metal, coat it with a paint stripper, then scrape it off with a straight edge.
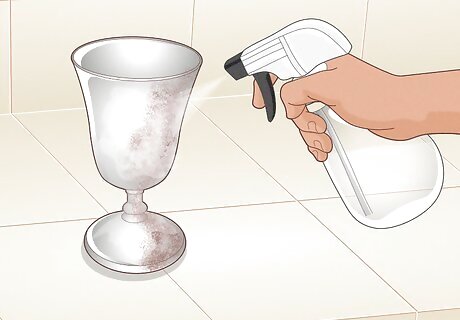
Mist the metal with the peroxide solution. For an even coat of rust, spray the metal up and down, trying to get the same amount of solution on each part. If you want a more patchy look, spray some areas more than others.
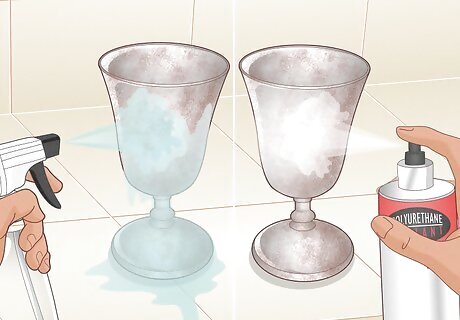
Gently rinse the rusted metal in water and then seal it. After a minute or two (or once the metal is rusted enough for you), spray it lightly with water to remove the excess salt. Let it dry completely (it may take up to 24 hours), then spray it with a clear coat of polyurethane sealant. Once the sealant dries, your rusting project is complete!
Rusting Galvanized Metal
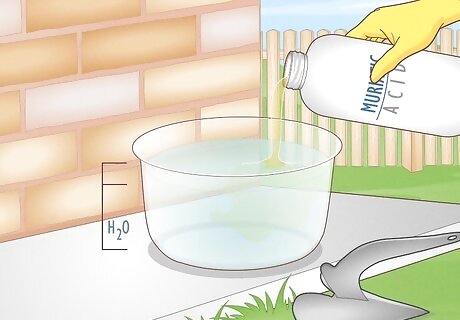
Make a solution of muriatic acid and water in a well ventilated space. Mix 3 parts water with 1 part muriatic acid in a plastic container large enough to submerge your metal in. Muriatic acid is highly corrosive, and zinc releases toxic fumes when it’s being dissolved, so work in a well ventilated area and wear goggles, gloves, and a respirator. Muriatic acid, also known as hydrochloric acid, can melt or soften plastic, so use a container you are okay with damaging, and neutralize the acid immediately after using it. Don’t overfill the container, or it could spill. Galvanized steel is coated in a thin layer of zinc to keep it from rusting, so you have to remove the zinc before you can work with the metal.

Put the metal in the liquid, then wait for the bubbles to slow down. Once zinc touches muriatic acid, it instantly starts to boil and bubble. When the bubbles die down, that means the zinc has been eaten away. Small pieces of metal only need a few minutes in the bath, but larger pieces take longer. If you’re still not sure if the metal is done, check the color. Galvanized metal is dark grey, while steel is silver and shiny.

Neutralize the metal in a solution of borax and washing soda. In a smaller container (still large enough to submerge your items) mix equal amounts of washing soda and borax into water until no more can be dissolved. Put your metal in the container and soak it for a minute. Washing soda and borax are both bases, which cancel out acids.
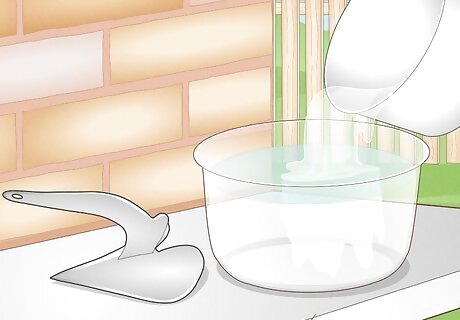
Mix the base solution into the acid solution to neutralize it. Once you’ve removed the zinc from all your metal, safely dispose of the acid by pouring the borax/baking soda solution into it. If there’s too much liquid, make a smaller batch of baking soda and water and slowly pour it into the acid. Always pour the base into the acid to avoid splashing yourself with something corrosive.
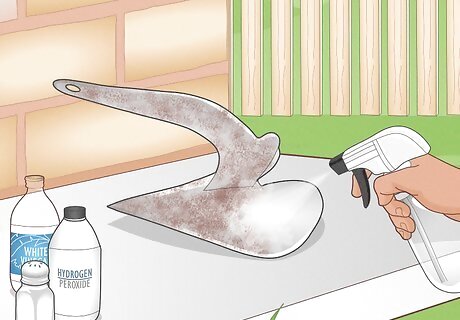
Spray the metal with a solution of vinegar, salt, and hydrogen peroxide. Once the zinc is gone, you can rust the metal using the first method above. Dry the metal off, then spray it with a mixture of 2 cups (470 mL) hydrogen peroxide, 4 US tbsp (59 mL) white vinegar, and 1.5 tsp (8.5 g) of table salt.



















Comments
0 comment