views
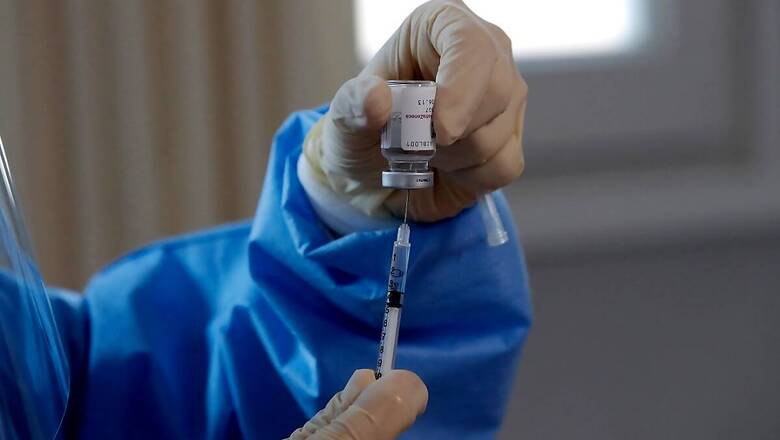
Of the almost 30 vaccine candidates currently under various phases of trials in India, Russian Sputnik V should be available for use in the next 4-6 weeks, Head of the Operations Research Group of the COVID-19 Task Force Dr NK Arora told CNBC-News18 on Tuesday.
“Zydus Cadila vaccine is next in line, should be available by end of May,” Dr Arora said adding that negligible to none allergic reactions have been observed in beneficiaries.
“Only 1 in 15,000 cases of anaphylaxis have been observed,” he said.
India launched the world’s largest vaccination drive on January 16 with two vaccines made in India – Covishield which is developed by AstraZeneca and Oxford University and is being produced by Pune’s Serum Institute of India and Hyderabad-based Bharat Biotech’s Covaxin.
In September last year, Hyderabad-based Dr Reddy’s Laboratories had partnered with the Russian Direct Investment Fund (RDIF) to conduct the clinical trials of Sputnik V and for its distribution rights in India. The vaccine is undergoing the phase 3 clinical trial in India.
Sputnik V, developed by the Gamaleya National Research Institute of Epidemiology and Microbiology, was registered by the Ministry of Health of Russia on August 11, 2020, and became the world’s first registered vaccine against COVID-19 based on the human adenoviral vector platform. Sputnik V has already received approval in 26 countries and has been administered to more than 20 lakh people worldwide.
Zydus Cadila’s vaccine candidate ZyCoV-D is being made in collaboration with the Department of Biotechnology. In January, DCGI gave its approval to the Ahmedabad-based drug firm to initiate Phase III clinical trials.
Read all the Latest News, Breaking News and Coronavirus News here

















Comments
0 comment