views
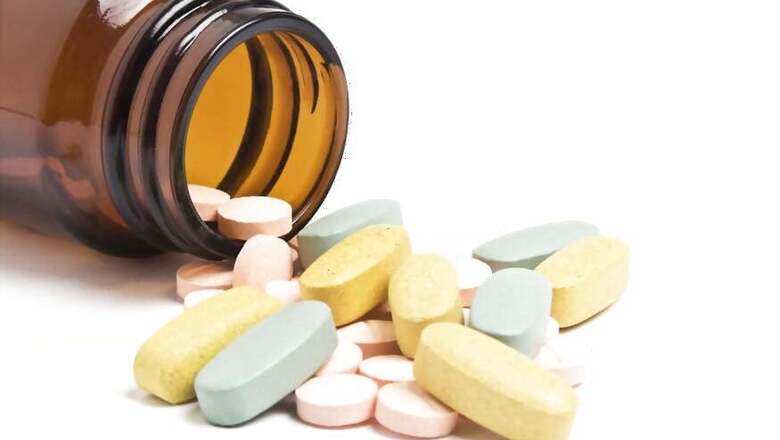
Strides Pharma Science Ltd on Wednesday said it has developed and commercialised Favipiravir antiviral tablets and stressed that the drug has demonstrated positive outcomes in Covid-19 treatment globally.
"Favipiravir is an antiviral medication that was initially developed to treat influenza in Japan. In February 2020, post the outbreak of Novel Coronavirus (Covid-19), Favipiravir was studied in China and several other countries as an experimental treatment of Covid-19. The drug has demonstrated positive outcomes, including a reduction in the duration of Covid-19 and improved lung conditions for the patients," the company said in a filing to the BSE.
The product is a generic version of Avigan of Toyama Chemical, Japan.
The filing said Strides is the first Indian company to have commenced export of Favipiravir tablets.
The company said it will immediately apply to Indian Drug Authorities to commence necessary studies and make the drug available to Indian patients expeditiously.
Strides has developed Favipiravir tablets in 400mg and 200mg strengths for convenient dosage administration.
The product is currently being exported to Gulf Cooperation Council (GCC) countries to treat patients under their treatment programme for Covid-19.
"Favipiravir tablets are being manufactured at Strides' flagship facility in Bangalore, India. The facility can produce up to 6 billion units of solid orals annually and is approved by the USFDA, MHRA, WHO, TGA, among others,"it said.
Strides has also entered into a preferred arrangement with a leading Indian API manufacturer for the supplies of Favipiravir API(active pharmaceutical ingredient).
"The partner has already commercialised the Favipiravir API from its USFDA, KFDA, PMDA and WHO approved manufacturing facility and has capabilities to manufacture the Favipiravir API from its Key Starting Material (KSM) in-house," it said.
Company's CEO and MD R Ananthanarayanan said, "We are pleased to be the First Indian company to develop and commercially launch Favipiravir tablets for the global markets. This development reinforces our commitment to play a substantial role in the society by bringing affordable and quality healthcare to millions of people around the globe."
Favipiravir has already demonstrated positive outcomes in several studies on Covid-19 patients, and the company is hopeful that the treatment regime with Favipiravir would brace up the fight against this virus, he said.
"Favipiravir is a complex drug to make, while we are manufacturing the tablets in-house, we are also excited to partner with the API manufacturer such that our supply chain remains secured up to the key starting material," he added.

















Comments
0 comment