views
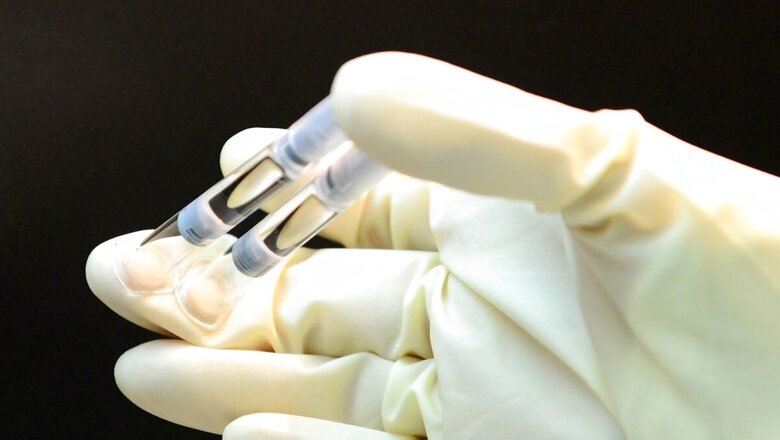
Biosimilars are generic equivalents of biologic drugs – a new class of medicines – and are more complex than chemical generics. Biologics include a range of products derived from large molecules such as monoclonal antibodies, gene therapies, hormones and enzymes. These substances are classified as biologics due to their production from living cells or organisms.
Several Indian pharmaceutical companies have been manufacturing and exporting biosimilars to the regulated market. They have a significant opportunity as per a CII report, which predicts that biopharma drugs will account for more than 40 percent of the global pharmaceutical market by 2030.
With this new target, it is imperative to ensure that the foundation is sufficiently robust, specifically the guidelines to approve biosimilars in India. Biosimilars provide a favourable advantage to patients since they are priced at a minimum of 50 percent lower than the original biologic medicines. In easier terms, you can call biosimilars a copycat version of biologics, which can be labelled as innovator or reference products.
Recently, several organisations working for greater public access to medicines and patients’ rights groups wrote to the central government requesting a change in the 2016 Guidelines on Similar Biologics (biosimilars) to facilitate “quicker access to quality biosimilars at an affordable rate”.
The health advocacy and patient groups have written the letter seeking a waiver on some requirements, calling them “a major barrier for the approval of biosimilars since they are both resource-intensive and time-consuming”. The pharma industry has also been asking for similar waivers.
The momentum of approvals can be understood by the fact that India is the only country with the largest number of approved biosimilars – 127 – ahead of Germany and the US. According to clinical trial protocols uploaded on the Clinical Trials Registry (CTRI), top 10 biosimilars in India have undergone Phase III trials on a sample size of patients ranging between 100 and 200.
While we mustn’t lose sight of the importance of affordability, we should also exercise caution to ensure that we don’t compromise the safety and effectiveness of drugs. Moreover, it is crucial to acknowledge that conquering the generic drug market was an entirely distinct endeavour undertaken during a different era.
Generics and biosimilars are significantly disparate products, warranting separate strategies and approaches. The government needs to ensure tighter controls before approval and periodic quality checks to ensure every batch – not only the initial batch – produced is showing consistent quality.
We are already observing the chaos unfolding in the generic medicines market due to lax controls and flawed regulatory practices. Errors in biosimilars, by nature, are much more likely.
Do doctors need more data and confidence?
While I spoke to more than half a dozen doctors to seek their opinions on using biosimilar and innovator products on patients in the real-world scenario, two doctors agreed to come on record.
A majority of doctors refused to talk on record fearing the label of “being biased” or “taking bribes from MNCs” or “anti-national”. In common, all doctors told me that while they do prescribe biosimilars considering the official approvals are in place and medicines are affordable, they personally put trust in drugs that have generated “a lot of data from clinical trials and testing” irrespective of the manufacturing firm.
Sample this: Dr CS Madhu, head of the oncology department at Lourdes Hospital in Cochin, believes that “we are in a deplorable situation where sanctions for biosimilars are given with loose guidelines”. He shared his reasons and case studies to prove his argument.
“Innovator drugs are backed by robust clinical data and randomised clinical trials, which are also performed with rivals of innovator molecules or dummy drugs,” Dr Madhu said.
Further, Dr Madhu, told me that “the Indian government has been giving a lot of approvals for biosimilars. We cannot adopt the strategy of generic drugs on biosimilars”.
“We are giving approvals on the basis of just preliminary studies, which are not comparable to the vast trial data available with innovator products,” he said, adding that “there is no effort to tighten the screws with an objective of manufacturing much finer products with greater efficacy”.
One of Dr Madhu’s patients – who was suffering from breast cancer – was put on an innovative medicine, he said. “That medicine became unavailable and, meanwhile, she was put on a copycat biosimilar. She started facing bleeding issues with the new drug. Later, her problem got resolved when she managed to get the innovator drug again,” he added.
Dr Madhu shared another instance where he was treating his friend’s wife who was fighting cancer. “Her tumour markers started rising till she was consuming the copycat. Later, when she managed to get the innovator drug in the UK, markers started coming down. This shows the efficacy difference,” he said.
Dr Poonam Patil, an oncologist at Bengaluru-based Manipal Hospital, shared a similar perspective. “There is no reason to say that biosimilars are inferior products but we will be more confident to prescribe these drugs if they will be approved with a vast set of data and clinical trials,” Dr Patil said. “These medicines are approved after testing on a very small group of patients whereas the innovator molecules are tested on thousands of patients.”
The oncologist added: “As a medical expert, I would put my trust more in those medicines that have undergone rigorous testing and have bigger studies.”
Dr Patil further said we need to accept that we cannot equate innovator drugs to biosimilars, which may be manufactured with loose guidelines. “The guidelines should be as rigorous and extensive for biosimilars as they are for biologics,” she said.
Relaxation of guidelines may further diminish confidence
According to the current guidelines on biosimilars, once the patent on an originator drug expires, rules require mandatory animal studies and comparative safety as well as efficacy studies or clinical trials to prove their clinical equivalence to the originator biologic drug.
To requests for waivers in existing approval protocols also give examples of multiple health agencies that have already allowed for the same, including the UK’s MHRA and the WHO. To critically examine the waiver request by a group of public health activists and patients’ bodies, I spoke to Dinesh Thakur, another public health activist focused on improving the quality of affordable medicines across the globe.
Thakur clarified the basis on which the UK’s MHRA, in 2021, waived off animal studies for the approval of biosimilars. He said modifications do not offer a “blanket” waiver. “They do it on a case-by-case basis,” he said.
He emphasised and quoted the MHRA guidance, which states: “Although each biosimilar development needs to be evaluated on a case-by-case basis, it is considered that, in most cases, a comparative efficacy trial may not be necessary if sound scientific rationale supports this approach.”
“Similarly, the WHO guidelines also do not advocate blanket waiver but on a case-by-case basis,” Thakur said.
Thakur quoted the guideline saying “comparative efficacy and safety trial will not be necessary if sufficient evidence of similarity can be drawn from other parts of the comparability exercise”.
“The request for a waiver has conveniently ignored the important aspects,” he said.
Misleading patients in the name of affordability
Merely due to the resource-intensive and time-consuming nature of trials, one cannot circumvent or bypass them. Bejon Misra, an international consumer policy expert and consumer advocate, also shared his strong opinion.
“I have always observed that it’s easy to mislead consumers in the name of affordability and lower prices,” he said.
Misra, who is part of several stakeholder committees with central and state governments, said, “The government held several stakeholder consultations and we told ICMR that we cannot dilute testing procedures for the sake of making them affordable.”
He said many of these Indian products are unable to gain approvals from overseas regulators, and they were being sold to Indian consumers because they cost less. “Don’t treat our consumers as guinea pigs, please!” he added.
“We have been asking Indian manufacturers why other regulators are not giving such aggressive approvals to our products but nobody is giving us answers,” Misra said.
He added: “In fact, it has been seen that Indian companies are tying up or setting up facilities abroad to develop biosimilars for pushing their approvals for exports.”
If we want to support patients, Misra said, “We need to ask for universal health coverage.”
What should be done instead of loosening guidelines?
Rather than seeking the loosening of guidelines, it would be more beneficial to advocate for mechanisms that give us medicines either by subsidies or insurance covers; but not those manufactured based on weak regulations.
Overall, by seeking lenient regulatory procedures, we are unjustly posing a disadvantage to Indian companies that produce exceptional biosimilars and possess the potential to elevate the country’s biosimilar market to new heights.
















Comments
0 comment